
CHEMICAL ACTIVITY SERIES SERIES
The activity series is used to determine how an element will react when it is kept in water, acid, or solutions containing different ions of different metals.Īn Activity series consists of lists of elements according to their decreasing order of reactivity. As we know that the highly reactive elements will replace the less reactive elements hence, you will get the products of the chemical reaction. Also, check the reaction of the elements if it is highly active or not. See where the elements are situated in the Activity Series. Step 1: Check the elements in the reaction. We need to understand the Activity Series to determine and predict the products of the reaction.

Some of the elements do not react at all these are the metals that are under hydrogen, like silver, gold, and platinum. Also, to visualize which metals take time to react to replace the hydrogen atoms like cobalt, nickel, tin, etc. The activity series of metals was developed so that it becomes easier for us to understand which metals are more reactive and which ones will react in any medium like sodium, magnesium, etc. The faster the reaction, the faster it loses electrons to form positive charges or positive ions. In general, the more reactive a metal is, the more vigorously it reacts with other metals elements. The reactivity series of metals is a chart listing metals in order of decreasing reactivity of the elements. The activity series is a set of different elements like metals and non-metals listed in decreasing order of their reactivity. It helps to determine the nature of the reaction and which element will displace the other element in a chemical reaction. The Activity Series is developed to determine the reaction of metals and non-metals elements with different elements in different conditions.
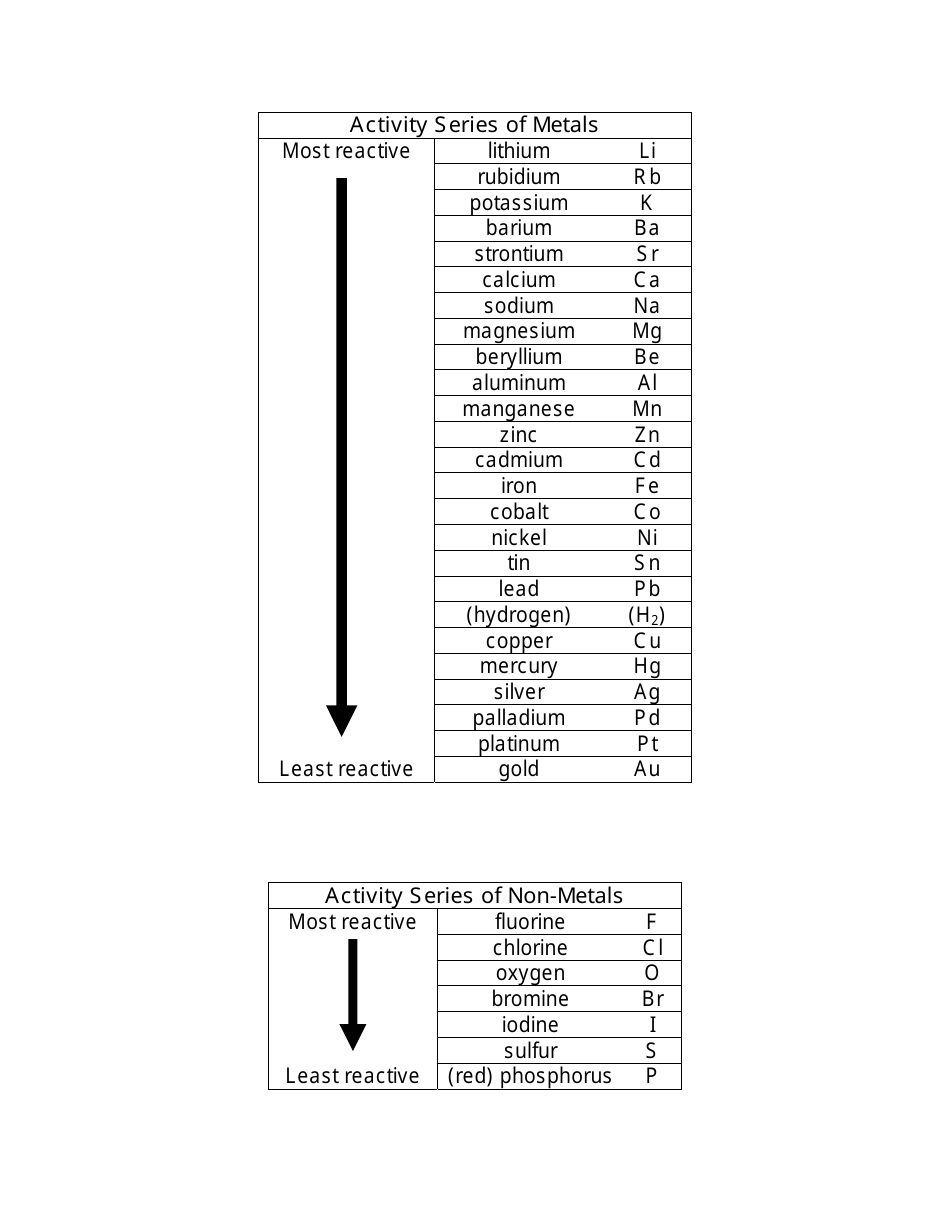
Well, it can be difficult to understand how an element will react and in which conditions hence, to know the reactivity of metals, the activity series is developed.
CHEMICAL ACTIVITY SERIES HOW TO
But, how to understand which elements will react with the other or which one will replace and take its place? We know due to some of the chemical reactions, all the elements react to form new compounds. Potassium is the most reactive, immediately produces purple sparks and flames.īe sure to use small pea-sized pieces of metal.Have you ever wondered why different metals and nonmetal elements react with each other? Why do some of the elements in the periodic table not react at all? All of these are very thoughtful questions. Be careful of larger chunks, which, due to internal air pockets, may take off like rockets from dish. Optional: Use hexane to rinse mineral oil from metal surface. The metal will scoot around on the surface of the water, producing bubbles of hydrogen and causing the phenolphthalein indicator to turn pink.


Three crystallizing dishes filled with waterĭrop a small piece of Li, Na or K into each dish.There are often microscopic pits in the metal causing the metal to launch. Do not do this experiment on the overhead.The metals react vigorously with water such that even water in the air is enough to ignite sodium and potassium.Store all metals under oil to protect from moisture.Increasing reactivity of group one metals is demonstrated. The resulting metal hydroxide solutions change the indicator from clear to pink. Lithium, sodium and potassium metals are added to water containing phenolphthalein indicator. Facility Announcements & Equipment Logbooks.Additional Info for Prospective Students.


 0 kommentar(er)
0 kommentar(er)
